Abstract

Background: Emicizumab is a bispecific monoclonal antibody that bridges factor IXa and X, substituting the cofactor function of FVIII, but artificially shortens aPTT and interferes with aPTT-based assays. The activated whole blood clotting time (ACT) is the preferred point-of-care test to monitor anticoagulation with high-dose heparin in cardiopulmonary bypass (CPB). While theoretically ACT may be susceptible to the effects of emicizumab as the intrinsic pathway is involved, in vitro human clinical data suggest that emicizumab does not interfere with the ACT. There has been little experience managing patients with hemophilia A on emicizumab during CPB, and in most cases it is not feasible to delay surgery to await clearance of emicizumab due its long half-life. Heparin concentration by Hepcon protamine titration is an alternative monitoring method.
Case: We describe the perioperative management of a patient with severe hemophilia A on maintenance emicizumab prophylaxis who underwent CPB with high-dose heparin monitored with both ACT and Hepcon. This 54-year-old male has a complex medical profile including Child Pugh B cirrhosis (baseline INR 1.5, platelets 100), Hepatitis C (treated), HIV on antiretroviral therapy (undetectable viral load), and remote portal vein thrombosis in the context of FEIBA and tranexamic acid use for a gastrointestinal bleed. He was diagnosed with Group B Streptococcus infective endocarditis. Despite optimal medical management, he developed aortic root abscess and aortic regurgitation necessitating operative intervention. A multidisciplinary team of hematology, cardiology, and cardiac surgery/anesthesia/intensive care deemed the risk of bleeding significant but not prohibitive and created a perioperative plan with liberal FVIII replacement followed by chromogenic FVIII levels postop (Table 1) to guide dosing.
A bioprosthetic valve was chosen to reduce the need for postoperative anticoagulation. Emicizumab prophylaxis was continued. Intra-operative heparin titration was performed by the Hepcon point-of-care assay, but concurrent ACT measurements were taken to investigate the effect of emicizumab. INR, fibrinogen, hemoglobin, and platelet levels were measured as per surgical protocol. Preop FVIII replacement with moroctocog alfa (Xyntha®) (60 U/kg) was administered to target a ~120% FVIII level. Tranexamic acid was administered intraoperatively as per surgical protocol. He underwent a successful aortic valve replacement, bovine patch reconstruction and aortic root repair on CPB pump for a total of 175 minutes with no excessive intraoperative bleeding. Table 1 summarises the perioperative course, point-of-care testing results, hemostatic therapy, and FVIII levels. Following administration of protamine at the end of CPB (ACT 135s, Hepcon 0 U/mL) there was persistent oozing from the sternum which lessened with surgical packing and additional Xyntha®, but ultimately the chest was closed in favour of medical management. Two hours post-op there was ~1600mL of blood loss via chest tubes despite a FVIII level of 89%, platelets 147, and INR 1.7. Fibrinogen concentrate, fresh frozen plasma, and platelets were given prior to a repeat OR for exploration. Bleeding was confirmed from the sternal bone and muscle, and hemostasis was achieved with cautery and sutures. The remainder of the post-op course was uneventful with no further bleeding on emicizumab prophylaxis and FVIII replacement for 14 days.
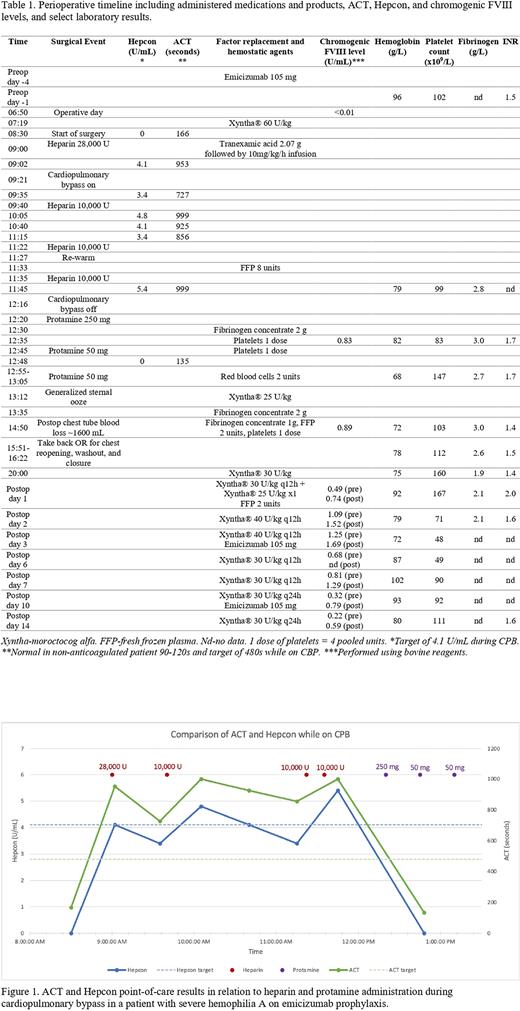
Discussion: The ACT and Hepcon results followed a similar trajectory over the operative course (Fig 1). Baseline and post-protamine ACT results were above normal reference range for non-anticoagulated patients (90-120s), and the intraoperative values were consistently above the target of 480s for CPB even when the Hepcon results fell below target (4.1 U/mL). The ACT results weren't artificially lowered with emicizumab, in fact elevated ACT values could have led to heparin underdosing risking circuit thrombosis. ACT is influenced by multiple variables including platelet count and function, antiphospholipids, factor deficiencies, temperature, and hemodilution. Therefore, the results may reflect derangements in one or more of these variables. We need further preclinical and clinical studies to better characterize the effect of emicizumab on ACT and identify the safest intraoperative monitoring strategy for patients on emicizumab requiring CPB surgery.
Disclosures
Sun:Sanofi/Sobi, Shire/Takeda: Honoraria. Goodyear:Alexion, Bayer, CSL Behring, Novo Nordisk, KVR Pharmaceuticals, Sanofi/sobi, Takeda: Honoraria. Poon:Bayer, CSL-Behring: Research Funding; Bayer, Bioverativ/Sanofi, CSL-Behring, KVR Pharmaceutical, Novo Nordisk, Pfizer, Roche, Sobi, Takeda: Honoraria. Rydz:BMS, Pfizer, Shire/Takeda: Honoraria, Speakers Bureau; NovoNordisk, Bayer, Agios: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal